Call08045476106
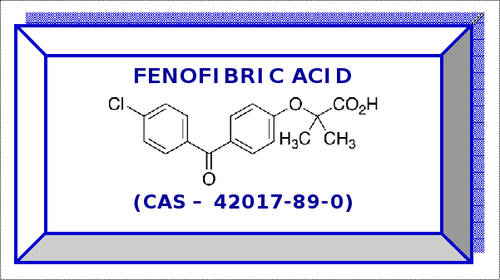
Fenofibric Acid
Product Details:
- Smell Odorless
- Appearance White to off-white powder
- Properties White to off-white crystalline powder, non-hygroscopic
- Density 1.32 Gram per cubic centimeter(g/cm3)
- Physical Form Powder
- Molecular Weight 318.75 g/mol
- Storage Store in cool, dry place, protected from light and moisture
- Click to View more
X
Fenofibric Acid Price And Quantity
- 1 Unit
- HDPE drums/bottles with double polyethylene liner
- Conforms to reference
- 99%
- Stable under recommended storage conditions
- Not available (decomposes prior to boiling)
- 90% 10 micron
- Meets ICH guidelines
- 10 ppm
- 0.5%
- 4.4
Fenofibric Acid Product Specifications
- 204-207C
- Non-poisonous
- Practically insoluble in water, soluble in methanol, ethanol, and DMSO
- White to off-white powder
- Active Pharmaceutical Ingredient
- Tasteless
- Odorless
- 255-579-9
- Used in formulation of lipid-lowering drugs
- Crystalline
- White to off-white crystalline powder, non-hygroscopic
- C17H15O4Cl
- 36 months
- 
- 29163990
- Not applicable / Neutral
- Store in cool, dry place, protected from light and moisture
- 318.75 g/mol
- Powder
- Pharmaceutical Grade
- 99%
- 1.32 Gram per cubic centimeter(g/cm3)
- Fenofibric Acid
- 42017-89-0
- HDPE drums/bottles with double polyethylene liner
- Conforms to reference
- 99%
- Stable under recommended storage conditions
- Not available (decomposes prior to boiling)
- 90% 10 micron
- Meets ICH guidelines
- 10 ppm
- 0.5%
- 4.4
Product Description
-
Fenofibric Acid CAS - 42017-89-0
PROPERTIES AND USES
White Crystalline material.
ASSAY : Min 99.0%%
Molar mass 360.831 g/mol
Starting material for Fenofibrate
Packaging Details: 25 KG / 50 KG BAGS / DRUMS
Superior Pharmaceutical Grade Quality
Fenofibric Acid stands out for its exceptional purity and compliance with international pharmacopeia standards. Stringent controls on heavy metals and residual solvents guarantee the integrity of each batch, while particle size distribution ensures applications in precise dosage forms. Each shipment includes a comprehensive certificate of analysis confirming identification (IR), assay (HPLC), and other specifications.
Safe and Stable Storage
For optimal stability and longevity, Fenofibric Acid should be stored in a cool, dry environment, away from direct light and moisture. Packaged in HDPE containers utilizing double polyethylene lining, the product remains protected from external contaminants and humidity. The compound maintains quality and stability throughout its 36-month shelf life when stored as recommended.
Applications and Usage
Mainly used in the pharmaceutical industry, Fenofibric Acid serves as a key ingredient in the formulation of drugs aimed at lowering lipid levels in patients. It is conveniently soluble in commonly used organic solvents such as methanol, ethanol, and DMSO, facilitating its integration into various pharmaceutical processes.
FAQs of Fenofibric Acid:
Q: How is Fenofibric Acid typically used in pharmaceutical applications?
A: Fenofibric Acid is primarily utilized in the development and manufacture of lipid-lowering medications. Its high purity and stability make it an ideal active ingredient in formulations targeting cholesterol management.Q: What storage conditions are recommended for maintaining the quality of Fenofibric Acid?
A: Fenofibric Acid should be stored in a cool, dry place, protected from light and moisture. Optimal stability is achieved with packaging in HDPE drums or bottles featuring double polyethylene liners.Q: What are the main benefits of using pharmaceutical-grade Fenofibric Acid?
A: This grade of Fenofibric Acid offers exceptional purity (99%%), stability, and compliance with stringent regulatory standards, ensuring reliable performance and safety in drug formulation.Q: What are the identification and purity verification methods for Fenofibric Acid?
A: Identification is confirmed via infrared (IR) spectrometry, while purity is verified using high-performance liquid chromatography (HPLC), both conforming to pharmacopeial standards.Q: Where is Fenofibric Acid commonly manufactured and supplied from?
A: Fenofibric Acid is manufactured, supplied, and distributed by trusted suppliers and wholesalers in India, catering to both domestic and international pharmaceutical companies.Q: Is Fenofibric Acid safe to handle in laboratory or manufacturing settings?
A: Yes, Fenofibric Acid is non-hygroscopic, non-poisonous, tasteless, and odorless, making it safe for standard handling in pharmaceutical environments when proper procedures are followed.Q: When does Fenofibric Acid typically decompose, and does it have a boiling or flash point?
A: Fenofibric Acid decomposes before reaching a boiling point, and there is no applicable flash point. It remains stable under recommended storage and handling conditions.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email



